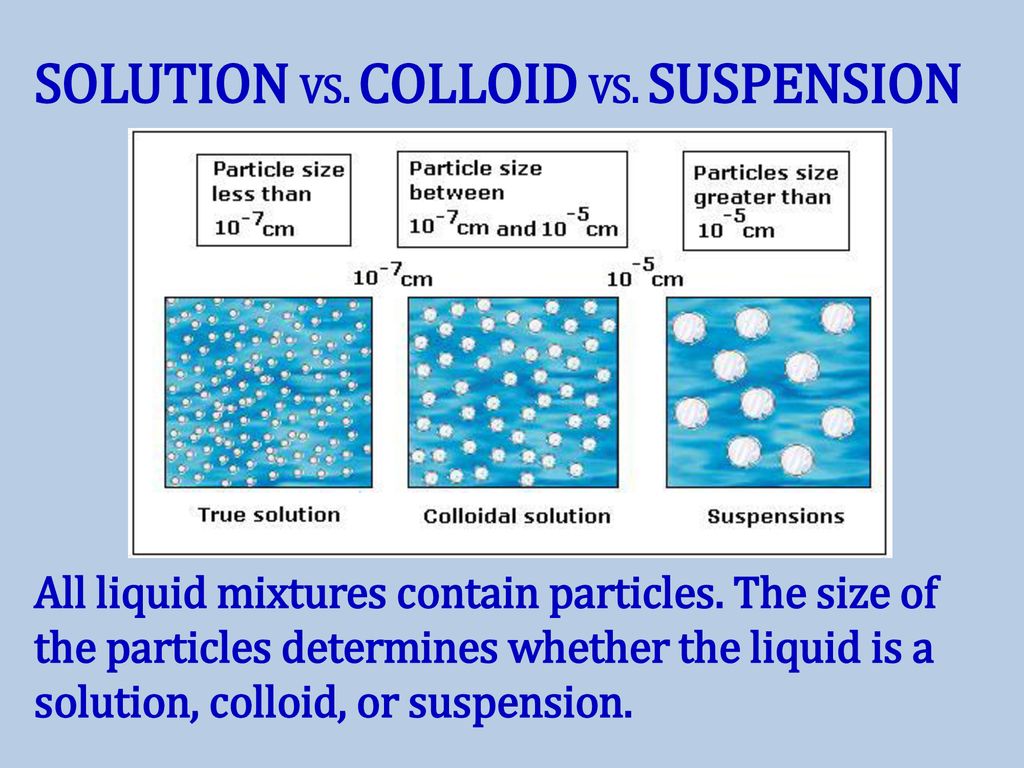
Colloid Solution Difference . a colloid is a mixture where one substance of microscopically dispersed insoluble particles is suspended throughout. summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. key differences between colloid and solution. difference between a colloid and a solution. Also, the solute and solvent constitute. A beam of light passing through a true. For the various dispersion types. colloids can be distinguished from solutions using the tyndall effect. the particles in a suspension are far larger than those of a solution, so gravity is able to pull them down out of the. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. A colloid is a mixture containing dispersed or suspended particles in the dispersion medium. The particle size in a solution is smaller than in a colloid,.
from slideplayer.com
The particle size in a solution is smaller than in a colloid,. the particles in a suspension are far larger than those of a solution, so gravity is able to pull them down out of the. summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. key differences between colloid and solution. Also, the solute and solvent constitute. A beam of light passing through a true. a colloid is a mixture where one substance of microscopically dispersed insoluble particles is suspended throughout. For the various dispersion types. colloids can be distinguished from solutions using the tyndall effect.
Solutions, Colloids, and Suspensions ppt download
Colloid Solution Difference summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. A colloid is a mixture containing dispersed or suspended particles in the dispersion medium. colloids can be distinguished from solutions using the tyndall effect. key differences between colloid and solution. Also, the solute and solvent constitute. A beam of light passing through a true. For the various dispersion types. a colloid is a mixture where one substance of microscopically dispersed insoluble particles is suspended throughout. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. the particles in a suspension are far larger than those of a solution, so gravity is able to pull them down out of the. summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. difference between a colloid and a solution. The particle size in a solution is smaller than in a colloid,.
From pediaa.com
Difference Between True Solution and Colloidal Solution Definition Colloid Solution Difference key differences between colloid and solution. Also, the solute and solvent constitute. colloids can be distinguished from solutions using the tyndall effect. summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. difference between a colloid and a solution. in this chapter, we will consider the nature of solutions, and examine factors that determine. Colloid Solution Difference.
From exopfanhq.blob.core.windows.net
Colloid Solution Description at Christine Veliz blog Colloid Solution Difference summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. colloids can be distinguished from solutions using the tyndall effect. key differences between colloid and solution. The particle size in a solution is smaller than in a colloid,. A beam of light passing through a true. difference between a colloid and a solution. the. Colloid Solution Difference.
From www.difference.wiki
True Solution vs. Colloidal Solution What’s the Difference? Colloid Solution Difference a colloid is a mixture where one substance of microscopically dispersed insoluble particles is suspended throughout. A beam of light passing through a true. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. For the various dispersion types. The particle size in a solution is smaller. Colloid Solution Difference.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Colloid Solution Difference colloids can be distinguished from solutions using the tyndall effect. difference between a colloid and a solution. summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. the particles in a suspension are far larger than those of a solution, so gravity is able to pull them down out of the. For the various dispersion. Colloid Solution Difference.
From pediaa.com
Difference Between Colloid and Solution Definition, Properties Colloid Solution Difference A beam of light passing through a true. summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. the particles in a suspension are far larger than those of a solution, so gravity is able to pull them down out of the. For the various dispersion types. difference between a colloid and a solution. a. Colloid Solution Difference.
From chemwiki.ucdavis.edu
Figure 1 Examples of a stable and of an unstable colloidal dispersion Colloid Solution Difference A colloid is a mixture containing dispersed or suspended particles in the dispersion medium. A beam of light passing through a true. summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. Also, the solute and solvent constitute. a colloid is a mixture where one substance of microscopically dispersed insoluble particles is suspended throughout. For the various. Colloid Solution Difference.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Colloid Solution Difference A beam of light passing through a true. colloids can be distinguished from solutions using the tyndall effect. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. The particle size in a solution is smaller than in a colloid,. summarize the principal distinguishing properties of. Colloid Solution Difference.
From cefjjtvg.blob.core.windows.net
Is Colloid A Homogeneous Solution at Anne Rojo blog Colloid Solution Difference a colloid is a mixture where one substance of microscopically dispersed insoluble particles is suspended throughout. summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. The particle size in a solution is smaller than in a colloid,. Also, the solute and solvent constitute. in this chapter, we will consider the nature of solutions, and examine. Colloid Solution Difference.
From askanydifference.com
Colloid vs Suspension Difference and Comparison Colloid Solution Difference The particle size in a solution is smaller than in a colloid,. a colloid is a mixture where one substance of microscopically dispersed insoluble particles is suspended throughout. key differences between colloid and solution. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. A colloid. Colloid Solution Difference.
From www.difference.wiki
Colloid vs. Solution What’s the Difference? Colloid Solution Difference a colloid is a mixture where one substance of microscopically dispersed insoluble particles is suspended throughout. key differences between colloid and solution. difference between a colloid and a solution. summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. the particles in a suspension are far larger than those of a solution, so gravity. Colloid Solution Difference.
From mungfali.com
Colloid Chart Colloid Solution Difference key differences between colloid and solution. Also, the solute and solvent constitute. For the various dispersion types. difference between a colloid and a solution. the particles in a suspension are far larger than those of a solution, so gravity is able to pull them down out of the. The particle size in a solution is smaller than. Colloid Solution Difference.
From scsscience9.blogspot.com
Is Matter Around Us Pure? Blog 6 Colloid Solution Difference For the various dispersion types. key differences between colloid and solution. A colloid is a mixture containing dispersed or suspended particles in the dispersion medium. A beam of light passing through a true. Also, the solute and solvent constitute. colloids can be distinguished from solutions using the tyndall effect. the particles in a suspension are far larger. Colloid Solution Difference.
From www.differencebetween.com
Difference Between Colloid and Emulsion Compare the Difference Colloid Solution Difference summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. The particle size in a solution is smaller than in a colloid,. For the various dispersion types. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. A colloid is a mixture containing dispersed or suspended. Colloid Solution Difference.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Colloid Solution Difference The particle size in a solution is smaller than in a colloid,. difference between a colloid and a solution. the particles in a suspension are far larger than those of a solution, so gravity is able to pull them down out of the. For the various dispersion types. key differences between colloid and solution. Also, the solute. Colloid Solution Difference.
From cegexkfz.blob.core.windows.net
Colloid Solutions Quizlet at Katrina Cohen blog Colloid Solution Difference in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. a colloid is a mixture where one substance of microscopically dispersed insoluble particles is suspended throughout. Also, the solute and solvent constitute. A colloid is a mixture containing dispersed or suspended particles in the dispersion medium. The. Colloid Solution Difference.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Colloid Solution Difference For the various dispersion types. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. difference between a colloid and a solution. key differences between colloid and solution. a colloid is a mixture where one substance of microscopically dispersed insoluble particles is suspended throughout. A. Colloid Solution Difference.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference Colloid Solution Difference A colloid is a mixture containing dispersed or suspended particles in the dispersion medium. difference between a colloid and a solution. the particles in a suspension are far larger than those of a solution, so gravity is able to pull them down out of the. in this chapter, we will consider the nature of solutions, and examine. Colloid Solution Difference.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID1980281 Colloid Solution Difference in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. A beam of light passing through a true. summarize the principal distinguishing properties of solutions, colloidal dispersions, and suspensions. The particle size in a solution is smaller than in a colloid,. Also, the solute and solvent constitute.. Colloid Solution Difference.